Definition
Continuous Spectrum
1. A continuous spectrum : A spectrum having no apparent breaks or gaps throughout its wavelength range is termed as continuous spectrum. It is produced by hot solid, liquid, or very dense gas.
Definition
Spectral series
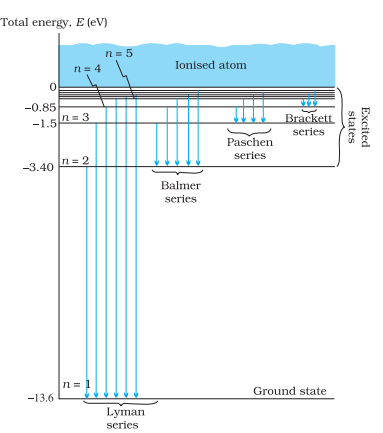
The emission spectrum of atomic hydrogen is divided into a number of spectral series, with wavelengths given by the Rydberg formula. These observed spectral lines are due to the electron making transitions between two energy levels in the atom.
Rydberg formula:
where Z=1 for hydrogen atom.
Rydberg formula:
where Z=1 for hydrogen atom.
Definition
Bohr's model

The Bohr model depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus similar in structure to the solar system, but with attraction provided by electrostatic forces rather than gravity.
- Bohr's first postulate was that an electron in an atom could revolve in certain stable orbits without the emission of radiant energy, contrary to the predictions of electromagnetic theory. According to this postulate, each atom has certain definite stable states in which it can exist, and each possible state has definite total energy. These are called the stationary states of the atom.
- Bohrs second postulate defines these stable orbits. This postulate states that the electron revolves around the nucleus only in those orbits for which the angular momentum is some integral multiple of h/2 where h is the Plancks constant (). Thus the angular momentum (L) of the orbiting electron is quantised. That is
- Bohrs third postulate incorporated into atomic theory the early quantum concepts that had been developed by Planck and Einstein.It states that an electron might make a transition from one of its specified non-radiating orbits to another of lower energy. When it does so, a photon is emitted having energy equal to the energy difference between the initial and final states. The frequency of the emitted photon is then given by
Definition
Bohr radius
The Bohr radius () is a physical constant, approximately equal to the most probable distance between the proton and electron in a hydrogen atom in its ground state.
Definition
Accelerated atomic electron spiral into nucleus
The model of the atom proposed by Rutherford assumes that the atom, consisting of a central nucleus and revolving electron is stable.object which moves in a circle is being constantly accelerated the acceleration being centripetal in nature. According to classical electromagnetic theory, an accelerating charged particle emits radiation in the form of electromagnetic waves. The energy of an accelerating electron should therefore, continuously decrease. The electron would spiral inward and eventually fall into the nucleus . Thus, such an atom can not be stable. As the electrons spiral inwards, their angular velocities and hence their frequencies would change continuously, and so will the frequency of the light emitted. Thus, they would emit a continuous spectrum, in contradiction to the line spectrum actually observed.
Formula
Electron orbits
According to Rutherford's nuclear model, the nucleus is at the center and electrons revolve in a circular orbit.
There must be a centripital force on the electron which enables it to move in a circular orbit.
Hence,
where, ze: charge on nucleus, e: charge on electron, r: orbital radius
Also
Hence solving this equation we get,
There must be a centripital force on the electron which enables it to move in a circular orbit.
Hence,
where, ze: charge on nucleus, e: charge on electron, r: orbital radius
Also
Hence solving this equation we get,
Definition
Orbital radius and electron velocity
The electrostatic force of attraction, between the revolving electrons and the nucleus provides the requisite centripetal force () to keep them in their orbits. Thus, for a dynamically stable orbit in a hydrogen atom
Thus the relation between the orbit radius and the electron velocity is
Thus the relation between the orbit radius and the electron velocity is
Definition
Quantum number of orbit
A 10 kg satellite circles earth once every 2 h in an orbit having a radius of 8000 km. Assuming that Bohrs angular momentum postulate applies to satellites just as it does to an electron in the hydrogen atom, find the quantum number of the orbit of the satellite.
Solution:
We have
Given m=10kg, , T=7200s
The quantum number of the orbit of satellite
Solution:
We have
Given m=10kg, , T=7200s
The quantum number of the orbit of satellite
Definition
Electron distribution in orbit or shells
According to atomic models, electrons move around the nucleus of atom in electron shells. Electrons in different shells have different energies. Each shell is represented by a number, , which is known as a shell number or energy level index.
The shell closest to the nucleus (and has the lowest energy) is called the -shell , the shell farther away (and has higher energy than K-shell) is called the -shell , in the similar way there is shell and shell .Bohr and Bury proposed the following rules for electron distribution.
Rule 1: The maximum number of electrons present in a shell is given by the formula , where 'n' is the shell number.
Rule 2: Each energy level or electron shell is further divided into sub shells. The maximum number of electrons that can be accommodated in each sub shell is 8.
Rule 3: Electrons cannot be filled in a given shell unless the inner shells are completely filled i.e., shells are filled in step wise manner.
The shell closest to the nucleus (and has the lowest energy) is called the -shell , the shell farther away (and has higher energy than K-shell) is called the -shell , in the similar way there is shell and shell .Bohr and Bury proposed the following rules for electron distribution.
Rule 1: The maximum number of electrons present in a shell is given by the formula , where 'n' is the shell number.
Rule 2: Each energy level or electron shell is further divided into sub shells. The maximum number of electrons that can be accommodated in each sub shell is 8.
Rule 3: Electrons cannot be filled in a given shell unless the inner shells are completely filled i.e., shells are filled in step wise manner.
Formula
Dependence of velocity, radius and energy on orbit of electron
Using Bohr's postulate, we can derive the expressions for radius(), velocity(), momentum and energy of an electron revolving in an orbit . We generally need to memorize the dependency of these variables on the orbit, n and atomic number, Z of the electron.
Let us see these dependencies.
Let us see these dependencies.
| BookMarks |
Page 12 Page 13 Page 14
0 Comments
Post a Comment