Definition
Principle and construction of cathode ray tube

The essential parts of a cathode ray tube make use of the following three processes:
1. Thermionic emission
2. Deflection of electron beam by the electric and magnetic fields
3. Fluorescence produced by the electron beam on a fluorescent screen
Construction: It consists of a long hollow evacuated glass tube containing the three main components: 1. The electron gun 2. The deflecting system 3. The fluorescent screen.
1. Thermionic emission
2. Deflection of electron beam by the electric and magnetic fields
3. Fluorescence produced by the electron beam on a fluorescent screen
Construction: It consists of a long hollow evacuated glass tube containing the three main components: 1. The electron gun 2. The deflecting system 3. The fluorescent screen.
Definition
Working of the electron gun, the deflecting system and the fluorescent screen in hot cathode ray tube
1. The electron gun: It is the part of cathode ray tube which gives out a fine beam of electrons. By varying the negative potential on the grid, the number of electrons striking the screen can be changed which changes the brightness of the pattern on the screen.
2. The deflecting system: It is the part of cathode ray tube which deflects the electron beam.
3. Deflection of electrons by the electric fields: When an electric field is produced between the two plates, the electron beam on entering the space between the plates, gets deflected towards the positive plate.
2. The deflecting system: It is the part of cathode ray tube which deflects the electron beam.
3. Deflection of electrons by the electric fields: When an electric field is produced between the two plates, the electron beam on entering the space between the plates, gets deflected towards the positive plate.
Definition
Uses of a cathode ray tube
A cathode ray tube is used mainly to convert electrical signal into a visual signal by applying the electric signal on the deflecting plates. Some of its usage are given below:
1. To determine the unknown frequency of an alternating potential by applying it on one pair of deflecting plates and comparing it with the known frequency of other alternating potential applied on other pair of plates.
2. To check the wave form of a varying electrical signal.
3. To measure a short time interval.
4. In television as a picture tube.
1. To determine the unknown frequency of an alternating potential by applying it on one pair of deflecting plates and comparing it with the known frequency of other alternating potential applied on other pair of plates.
2. To check the wave form of a varying electrical signal.
3. To measure a short time interval.
4. In television as a picture tube.
Definition
Photon
The particles of light are photons. Its properties are:
- A photon always travel at a speed in vaccum.
- The rest mass of a photon is zero.
- Each photon has a definite energy and linear momentum.
- During collisions, the number of photons may not be conserved.
- If the intensity of a given wavelength is increased, there is an increase in the number of photons crossing a given area in a given time. The energy of each photon remains the same.
Definition
Rutherford's nuclear model of atom
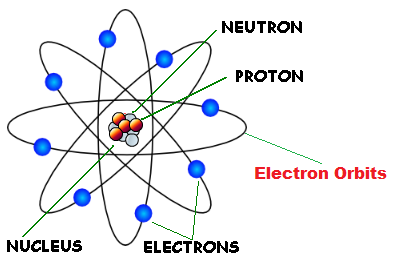
In Rutherford's nuclear model of the atom, the entire positive charge and most of the mass of the atom are concentrated in the nucleus with the electrons some distance away. The electrons would be moving in orbits about the nucleus just as the planets do around the sun. Rutherford's experiments suggested the size of the nucleus to be about m to m.
Definition
Kinetic,potential and total energy of electron
The kinetic energy (K) and electrostatic potential energy (U) of the electron
in hydrogen atom are
(The negative sign in U signifies that the electrostatic force is in the r direction.) Thus the total energy E of the electron in a hydrogen atom is
in hydrogen atom are
(The negative sign in U signifies that the electrostatic force is in the r direction.) Thus the total energy E of the electron in a hydrogen atom is
Definition
Thomson's model

Thomson's model represented an attempt to consolidate the known properties of atoms at the time: 1) electrons are negatively-charged particles and 2) atoms are neutrally-charged.
The model suggested alpha particles passing through the plum pudding model of the atom with negligible deflection.
The model suggested alpha particles passing through the plum pudding model of the atom with negligible deflection.
Example
Solve problem on cathode ray tube
Example: A cathode ray tube has a potential difference of volt between the cathode and the anode. Find the speed of cathode rays.
Solution:
The kinetic energy of electrons is:
The potential between cathode and anode is V hence,
Solution:
The kinetic energy of electrons is:
The potential between cathode and anode is V hence,
| BookMarks |
Page 12 Page 13 Page 14
0 Comments
Post a Comment