Definition
Atomic mass unit
Atomic mass unit (u) is defined as of the mass of the carbon
atom. According to this definition
atom. According to this definition
Example
Use the relationship between radius of a nucleus and its mass number
Example: Find the radius of the nucleus of an atom of mass number 216 (R 1.2 fermi).
Solution:
Solution:
Definition
Definition and importance of valency
The valence or valency of an element is a measure of its combining power with other atoms when it forms compounds or molecules.
When two atoms approach each other and react with each other, it is their outer shells that come into contact first, and it is therefore the electrons in their outer shells that are normally involved in any chemical reaction. So it is the number of electrons in an atoms outer shell that determines, to a large extent, how that element will react chemically.
Scientist concluded that when an element reacts to form compounds their atoms must be combining in such way that they can attain the stable electron distribution of noble gases or inert gases. An atom can achieve an octet by two ways, one by transfer of electrons and other by sharing of electrons. Both the processes result in the formation of bonds between atoms.
When two atoms approach each other and react with each other, it is their outer shells that come into contact first, and it is therefore the electrons in their outer shells that are normally involved in any chemical reaction. So it is the number of electrons in an atoms outer shell that determines, to a large extent, how that element will react chemically.
Scientist concluded that when an element reacts to form compounds their atoms must be combining in such way that they can attain the stable electron distribution of noble gases or inert gases. An atom can achieve an octet by two ways, one by transfer of electrons and other by sharing of electrons. Both the processes result in the formation of bonds between atoms.
Definition
Atomic Energy in India
Bhabha Atomic Research centre [BARC] is the major centre for research and development work in atomic energy in India. The first atomic power station in India called Tarapur atomic power station started working in 1969 in maharashtra.
Indias uranium supply comes mainly from Jaduguda mines in Bihar. India also imports enriched uranium from developed countries.
Kota (Rajasthan), Kalpakkam (Tamil Nadu), Kaiga (Karnataka) are the places where nuclear power reactors are functioning.
Indias uranium supply comes mainly from Jaduguda mines in Bihar. India also imports enriched uranium from developed countries.
Kota (Rajasthan), Kalpakkam (Tamil Nadu), Kaiga (Karnataka) are the places where nuclear power reactors are functioning.
Definition
Describe construction and working of Geiger - Muller counter Experiment
Construction and Working:
A Geiger counter consists of a Geiger-Mller tube, the sensing element which detects the radiation, and the processing electronics, which displays the result. The Geiger-Mller tube is filled with an inert gas such as helium, neon, or argon at low pressure, to which a high voltage is applied. The tube briefly conducts electrical charge when a particle or photon of incident radiation makes the gas conductive by ionization. The ionization is considerably amplified within the tube by the Townsend discharge effect to produce an easily measured detection pulse, which is fed to the processing and display electronics. This large pulse from the tube makes the G-M counter relatively cheap to manufacture, as the subsequent electronics is greatly simplified. The electronics also generates the high voltage, typically 400600 volts, that has to be applied to the Geiger-Mller tube to enable its operation.
A Geiger counter consists of a Geiger-Mller tube, the sensing element which detects the radiation, and the processing electronics, which displays the result. The Geiger-Mller tube is filled with an inert gas such as helium, neon, or argon at low pressure, to which a high voltage is applied. The tube briefly conducts electrical charge when a particle or photon of incident radiation makes the gas conductive by ionization. The ionization is considerably amplified within the tube by the Townsend discharge effect to produce an easily measured detection pulse, which is fed to the processing and display electronics. This large pulse from the tube makes the G-M counter relatively cheap to manufacture, as the subsequent electronics is greatly simplified. The electronics also generates the high voltage, typically 400600 volts, that has to be applied to the Geiger-Mller tube to enable its operation.
Definition
Geiger Marsden experiment
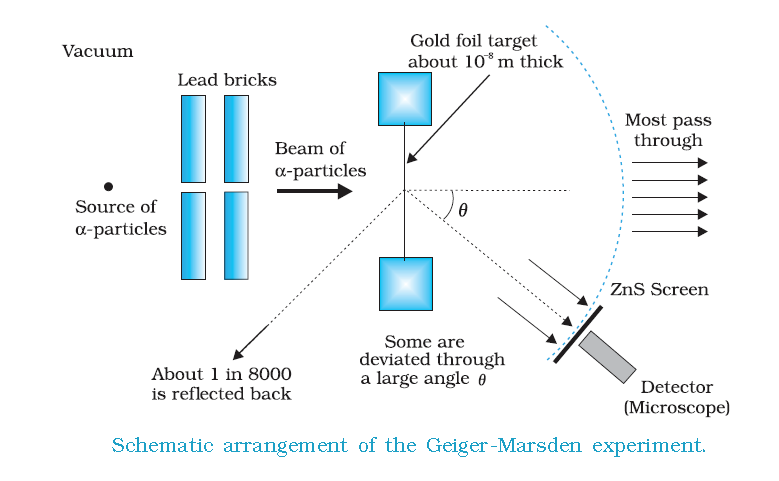
Geiger and Marsden performed a series of experiments where they pointed a beam of alpha particles at a thin foil of metal and measured the scattering pattern by using a fluorescent screen. They spotted alpha particles bouncing off the metal foil in all directions, some right back at the source.Obviously, those particles had encountered an electrostatic force far greater than Thomson's model suggested they would, which in turn implied that the atom's positive charge was concentrated in a much tinier volume than Thomson imagined.When Geiger and Marsden shot alpha particles at their metal foils, they noticed only a tiny fraction of the alpha particles were deflected by more than . Most just flew straight through the foil. This suggested that those tiny spheres of intense positive charge were separated by vast gulfs of empty space.
| BookMarks |
Page 12 Page 13 Page 14
0 Comments
Post a Comment