Definition
Lenard's experiment
Wilhelm Hallwachs and Philipp Lenard investigated the phenomenon of photoelectric emission in detail during 1886-1902.
Their observations can be summarised as below:
Their observations can be summarised as below:
- When light is incident on a metal plate, it may cause the electrons to come out of the metal.
- Hallwachs and Lenard also observed that when the light fell on the emitter plate, no electrons were emitted at all when the frequency of the incident light was smaller than a certain minimum value. This frequency is called threshold frequency.
- He also observed that different metals have different threshold frequencies.
Definition
Hertz's observation
The phenomenon of photoelectric emission was discovered in 1887 by Heinrich Hertz, during electromagnetic wave experiments.He observed that when light falls on a metal surface, some electrons near the surface absorb enough energy from the incident radiation to overcome the attraction of the positive ions in the material of the surface.
Definition
Effect of potential on photocurrent
Photoelectric current is zero when the stopping potential is sufficient to repel even the most energetic photoelectrons with the maximum kinetic energy so that
For a given frequency of the incident radiation, the stopping potential is independent of its intensity.
For a given frequency of the incident radiation, the stopping potential is independent of its intensity.
Definition
Dependence of intensity on photocurrent
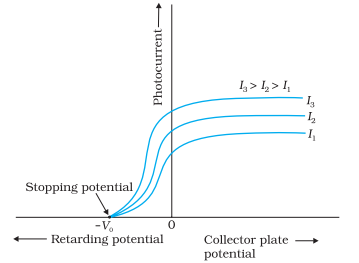
When the intensity of light is increased the saturation current also increases. It is shown in the figure given here. let the intensities be in the order
Then the saturation current
Then the saturation current
Definition
Dependence of stopping potential of light on frequency of light

For a particular frequency of incident radiation, the minimum negative (retarding) potential given to the plate A for which the photocurrent stops or becomes zero is called the cut-off or stopping potential.We now take three different lights at different frequencies , , but of same intensity. More the frequency of light, more negative potential is needed to stop the photocurrent. Hence the stopping potential increases. Hence as shown in the figure, the magnitude > >
Definition
Dependence of photo current on frequency of light
The saturation current depends on the intensity of light, not on frequency. Hence no matter how much is the frequency for the same intensity of light, the saturation photocurrent will be same.
Definition
Particle and wave nature of light
Dual nature of light:
- Sometimes it behaves like a particle (called a photon), which explains how light travels in straight lines
- Sometimes it behaves like a wave, which explains how light bends (or diffracts) around an object
Example
Problems on Millikan's drop experiment

In Millikan's oil drop experiment a fine mist of oil droplets was sprayed into a chamber above the plates. The oil was of a type usually used in vacuum apparatus and was chosen because it had an extremely low vapour pressure. Ordinary oil would evaporate under the heat of the light source causing the mass of the oil drop to change over the course of the experiment. Some oil drops became electrically charged through friction with the nozzle as they were sprayed. Alternatively, charging could be brought about by including an ionising radiation source (such as an X-ray tube). The droplets entered the space between the plates and, because they were charged, could be made to rise and fall by changing the voltage across the plates. It was found that the charge on an oil droplet was always an integral multiple of an elementary charge, . Thus, it was established that electric charge is quantized and from the values of charge (e) and specific charge (e/m, the mass (m) of the electron could be determined.
Example: In a Millikans oil drop experiment, an oil drop of mass , carrying a charge remains stationary between two plates separated by a distance of 5 mm. Given ; find the voltage that must be applied between the plates.
Solution:
Let the voltage applied by ,
According to equilibrium eqn.,
Example: In a Millikans oil drop experiment, an oil drop of mass , carrying a charge remains stationary between two plates separated by a distance of 5 mm. Given ; find the voltage that must be applied between the plates.
Solution:
Let the voltage applied by ,
According to equilibrium eqn.,
Example
Example on maximum velocity of photoelectron
then The work function of a metal surface is 1 eV. A light of wavelength 3000 is incident on it. The maximum velocity of the photoelectrons is found as:The maximum K.E with which electrons comes out,
Formula
Formula for maximum kinetic energy
Let us say a metal is irradiated with a photon of frequency . Let this frequency will be equal to the threshold frequency of light. Let the work function of the metal be . The energy of the photon will be .
As we know, some of the electrons may come out of the metal surface. What will be the energy of this photoelectron ejected?
Zero. why?
As we know, some of the electrons may come out of the metal surface. What will be the energy of this photoelectron ejected?
Zero. why?
- Because the energy given to the photoelectron is spent to overcome the work function of the metal.
- What if the light of threshold frequency is replaced with light of frequency more than the threshold frequency?Photoelectrons will emit in this case also. What will be their energy? Some amount of the energy will be lost to overcome the work function of the metal. The rest of the energy may be spent in the collision with other electrons.And the energy still left after this process is converted into kinetic energy of the electron.
- Let us say some of the electrons don't lose this extra energy. These electrons will come out with maximum kinetic energy. What will this energy be?
- It will be the energy of the photon minus the work function. Or
| BookMarks |
0 Comments
Post a Comment