Definition
Boyle's Law
The absolute pressure exerted by a given mass of an ideal gas is inversely proportional to the volume it occupies if the temperature and amount of gas remain unchanged within a closed system.
Definition
Dalton's Law of partial pressure
Dalton's Law states that in a mixture of non-reacting gases, the total pressure exerted is equal to the sum of the partial pressures of the individual gases.
Example
Use of Gay Lussac's Law
Q. Find the temperature in Celsius needed to change the pressure of liters of a gas that has a pressure of at to standard pressure. Standard pressure is .
Sol : First, we have to convert to Kelvin
From Gay Lussac's law, we know that
( at constant volume )
............. (1)
Here,
Putting the above values in equation (1), we will get,
In order to get temperature in celsius, we will have to subtract by ,
Sol : First, we have to convert to Kelvin
From Gay Lussac's law, we know that
( at constant volume )
............. (1)
Here,
Putting the above values in equation (1), we will get,
In order to get temperature in celsius, we will have to subtract by ,
Law
Introduction to Gay-Lussac's Law

Gay-Lussac's Law :Gay-Lussac's Law states that at constant volume for a fixed amount of gas, the pressure is directly proportional to its temperature in Kelvin.
Definition
Real gas equation
Real gases are often modeled by taking into account their molar weight and molar volume,
where is the pressure, is the temperature, is the ideal gas constant, and is the molar volume. and are parameters that are determined empirically for each gas, but are sometimes estimated from their critical temperature and critical pressure using these relations:
The compressibility factor (Z), also known as the compression factor, is the ratio of the molar volume of a gas to the molar volume of an ideal gas at the same temperature and pressure.
where is the pressure, is the temperature, is the ideal gas constant, and is the molar volume. and are parameters that are determined empirically for each gas, but are sometimes estimated from their critical temperature and critical pressure using these relations:
The compressibility factor (Z), also known as the compression factor, is the ratio of the molar volume of a gas to the molar volume of an ideal gas at the same temperature and pressure.
Diagram
compressibility factor vs pressure graph

Definition
Compressibility factor
There are three regimes that affect the compressibility factor:
- The value of Z tends toward 1 as the gas pressure approaches 0, where all gases tend toward ideal behavior
- The value of Z is less than 1 at intermediate pressures because the intermolecular forces of attraction cause the actual volumes to be less than the ideal values
- The value of Z is greater than 1 and ultimately tends toward infinity at high pressures because the intermolecular repulsive forces cause the actual volumes to be greater than the ideal values
Definition
Define and calculate pressure coefficient in gases
- A pressure coefficient is a dimensionless number which describes the relative pressures throughout a flow field in fluid dynamics.
- It is useful in aerodynamics and hydrodynamics.
- Every point in a fluid flow field has its own unique pressure coefficient,
- Consider a given mass of a gas is heated through 1 C at constant volume. Now the ratio of the increase in pressure to the original pressure at 0 C is defined as the pressure coefficient.
- If and be the pressures of a given mass of a gas at T C and 0C respectively, then
Definition
Introduction to Avogadro's Number
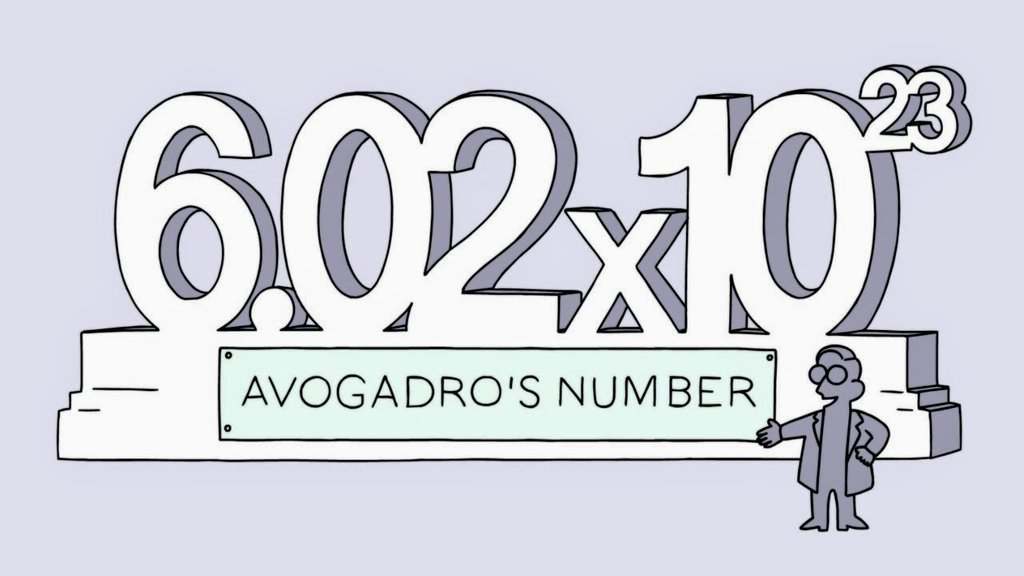
- Avogadro's number is the number of particles found in one mole of a substance. It is the number of atoms present in exactly 12 grams of carbon-12. Its value is approximately particles per mole which is determined experimentally. It is a dimensionless quantity.
| BookMarks |
0 Comments
Post a Comment